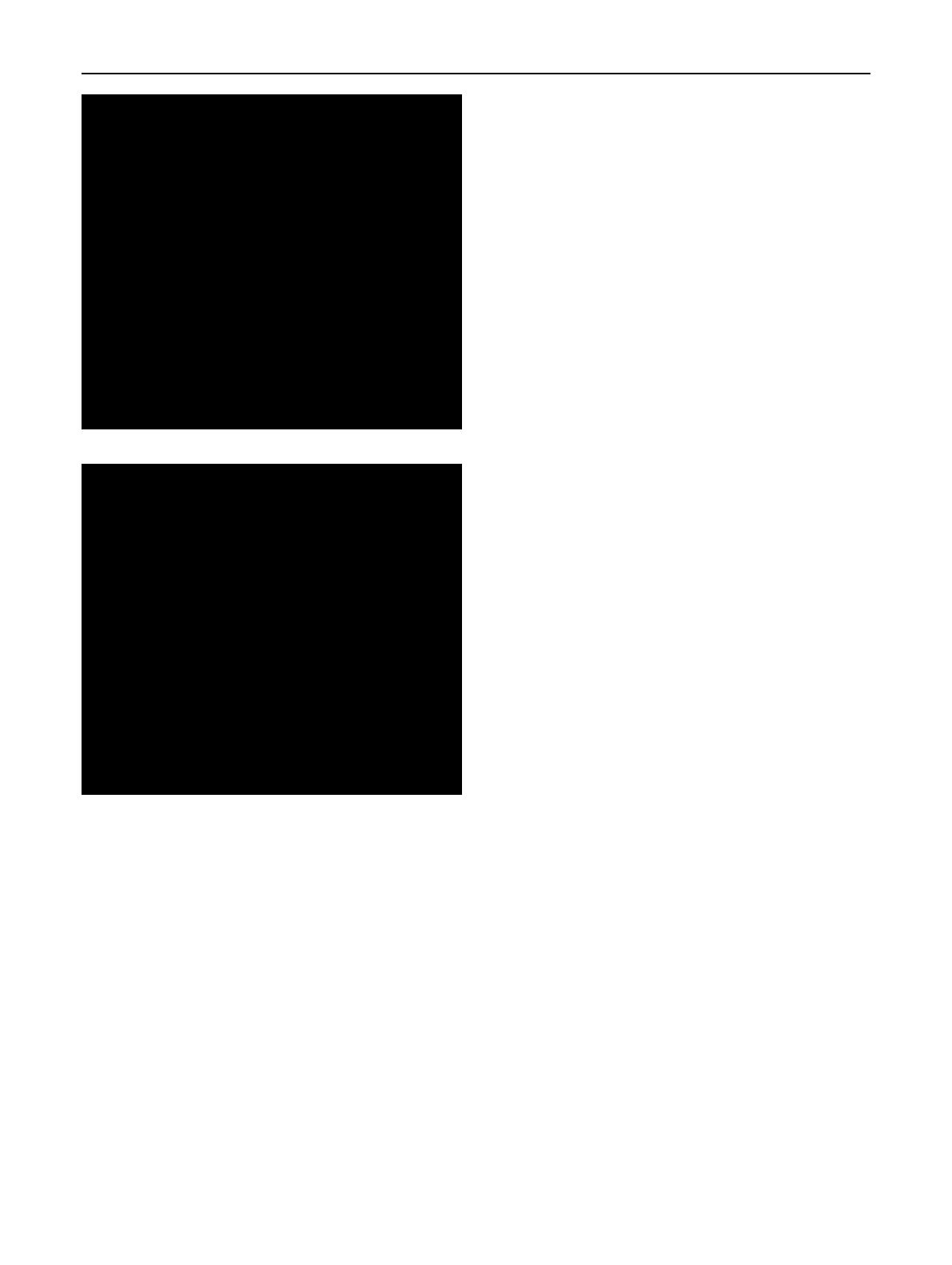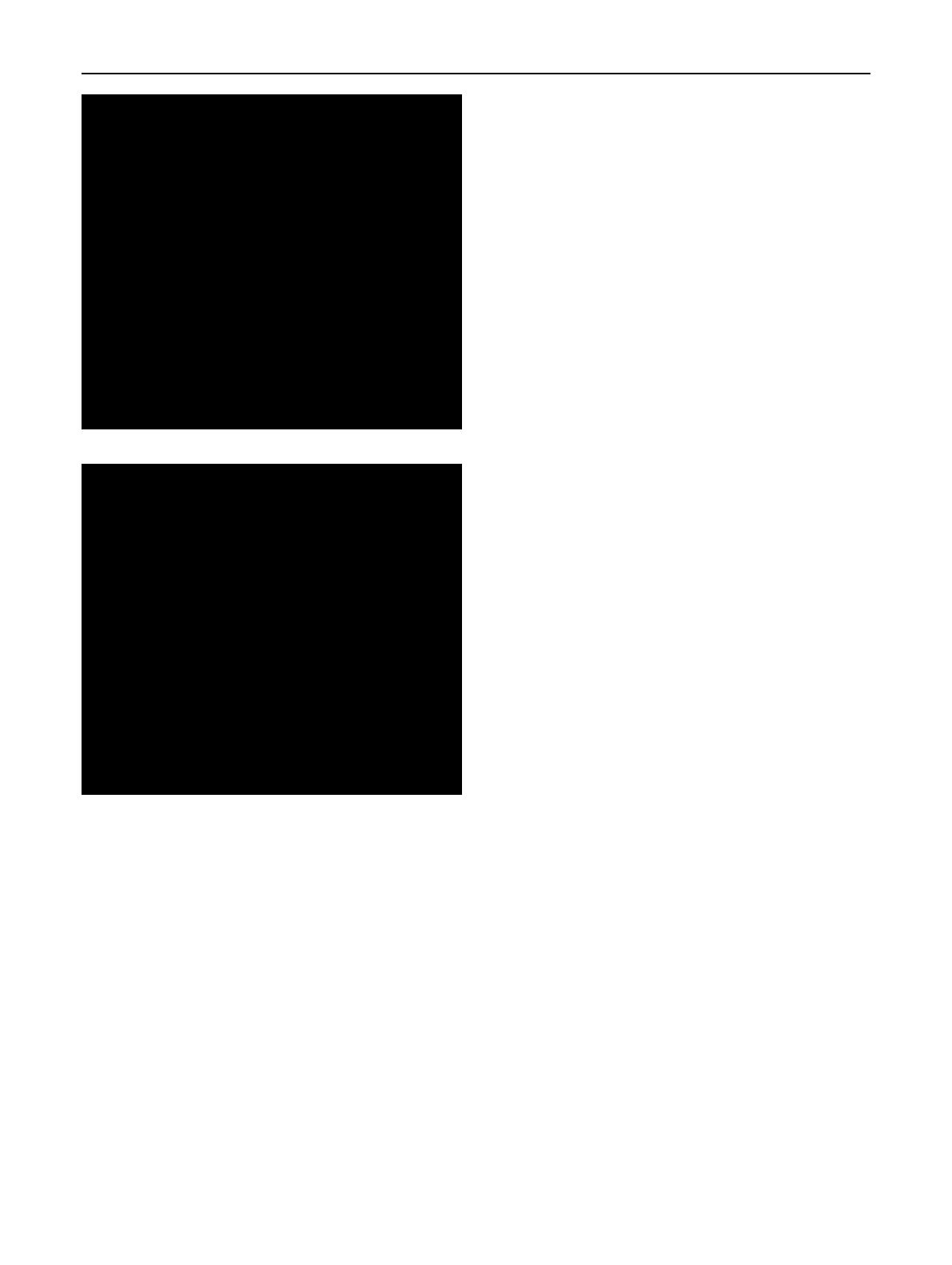
Figure 2 is presented as a simple example of recrystal-
lization annealing. Each of the many nonferrous metals and
metal alloy combinations has unique annealing character-
istics which produce a unique graph of data. In many
instances, adjustment may be required in the annealing
time and/or temperature to attain desired grain size.
Basic Requirements for Hardening by Heat Treatment
As stated above, practically all nonferrous alloys respond
readily to annealing treatments, but only a relatively small
portion of the total number respond signicantly to hard-
ening by heat treatment. With the exception of titanium,
common high-use aluminum, copper, and magnesium
alloys, are not allotropic; thus, they do not respond in the
same way as steels when subjected to heating and cooling
treatments.
At one time, technologists thought that ancient civili-
zations had developed a method for hardening copper
alloys that had become a lost art. Copper articles removed
from tombs showed a relatively high degree of hardness.
However, it was proved that the impurities in these copper
articles were responsible for their hardness. This hardness
had evidently developed over a period of centuries by the
precipitation mechanism rather than intentionally induced
by the people who made them.
Solubility and Insolubility
In many instances, two or more metals, alloyed above their
melting temperature, are completely soluble in each other,
which continues through the solid solution range. For
example, copper and tin alloy readily to form an entire
family of bronzes. For these alloys, the solid solutions
that form at elevated temperature remain completely stable
at room temperature or below. Such an alloy, therefore, can
be hardened only by cold working.
On the other hand, many alloys contain phases or con-
stituents that are readily soluble at elevated temperature, but
are far less soluble or insoluble at room temperature, which
is the basic requirement for hardening by heat treatment.
General Heat Treating Procedures
Alloys with the characteristics described above are harde-
nable at least to some degree. The metallurgical principles
responsible for this phenomenon are the same (or at least
closely related) for all of the nonferrous alloys. Likewise,
methods of processing are basically the same. However,
temperature and time cycles cover a wide range, depending
not only on alloy composition but also on whether the alloy
is in the wrought or cast condition.
As a rule, the highest possible increase in mechanical
properties for a given alloy is accomplished in two distinct
heating operations. First, the alloy is heated to a tempera-
ture just below (say at least 50 For28 C) its solidus
(beginning of melting) temperature. Holding time at tem-
perature depends on the solubility of the various phases and
whether the alloy is wrought or cast. It must be considered
that the primary purpose of this operation is to affect a solid
homogeneous solution of all phases. Consequently, this
part of the operation is commonly known as solution
treating or homogenizing.
Because the structures of castings are relatively heter-
ogeneous, a much longer time (at least twice as long) is
required at the solution treating temperature compared to a
wrought product of similar composition.
Fig. 2 Hardness as a function of annealing temperature for pure
copper and a Cu5Zn alloy, using three different annealing times.
Both materials were originally cold rolled at 75 F (25 C) to a 60%
reduction in thickness. Source Heat Treatment, Structure and
Properties of Nonferrous Alloys, p 29, 1982
Fig. 1 Schematic presentation of cold rolling a copperzinc alloy
(60% reduction). Hardness was 78 HRB before reduction, which
increased to 131 HRB after reduction. Source Heat Treatment,
Structure and Properties of Nonferrous Alloys, p 22, 1982
Metallogr. Microstruct. Anal. (2013) 2:190195 191
123